The UCSC CSC’s most high end dispenser is its acoustic dispenser.
The UCSC CSC’s most high end dispenser is its acoustic dispenser.
Basic information:
- Make/Model: Beckman Coulter Labcyte Echo 650
- What makes it special compared to other dispensers: it dispenses very small volumes very accurately without using tips or pins (contactless) and also measures the volume in the source and destination well.
- Capabilities in brief: dispenses liquid from a 384 or 1536 well Echo-qualified microplate to a variety of destination plates using sound waves to create small droplets. Transfers to one well at a time, a programmable volume, in any plate map.
- For high throughput: integrated on an Explorer workstation. (Or you can use it stand-alone walk-up as well.)
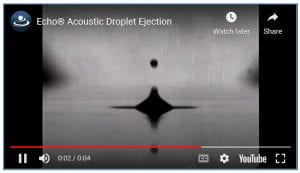 Think of the ripples in the glass of water in Jurassic Park (video clip here) or water on a speaker (like in a certain Justin Bieber music video). The effect of sound on liquid is a tangible phenomenon, and was first scientifically described in the 1920s. Putting it to use, however, is another question. The first commercial development was by Xerox and IBM for ink printers. More recently, it was used to develop liquid dispensers by Labcyte and EDC Biosystems, both now owned by Beckman Coulter, who sells the technology as Echo dispensers – watch a movie of the water droplet forming inside the Echo by clicking here or to the right. For more information on the history, see this PDF.
Think of the ripples in the glass of water in Jurassic Park (video clip here) or water on a speaker (like in a certain Justin Bieber music video). The effect of sound on liquid is a tangible phenomenon, and was first scientifically described in the 1920s. Putting it to use, however, is another question. The first commercial development was by Xerox and IBM for ink printers. More recently, it was used to develop liquid dispensers by Labcyte and EDC Biosystems, both now owned by Beckman Coulter, who sells the technology as Echo dispensers – watch a movie of the water droplet forming inside the Echo by clicking here or to the right. For more information on the history, see this PDF.
Acoustic dispensers use Acoustic droplet ejection (ADE) technology.
An ultrasonic pulse, delivered by a piezoelectric transducer, is transmitted through a water column (the coupling fluid) and focused by a synthetic lens at the source liquid surface, causing the liquid to form first a mound, then ripples, until it gives off a tiny droplet. The size of the droplet depends on the frequency of the sound – ours makes 2.5 nl droplets. Thus, sound energy is converted to kinetic energy in the upwardly moving droplet. The droplet hits the inverted destination plate well (usually dry) and is held there by surface tension. Watch this informational YouTube video for a demonstration of how ADE technology works inside the Echo instrument.
For more information, check out the SLAS Technology special issue on acoustic dispensing: Volume 21 issue 1, 2016.
The acoustic dispensing process is:
- contactless – no tubes or nozzles, therefore nothing to wash, preserved sample integrity, no chemicals from labware entering the specimen, no sample/potency loss from adhering to tips, no cross-contamination between samples, no consumables (i.e. tips) used up.
- gentle – it won't denature proteins, damage DNA, or kill cells during their transfer.
- smart – not only moves the liquid, but also measures the volume of the liquid.
- accurate – especially for small volumes.
For more information, check out the Beckman Coulter product info website.
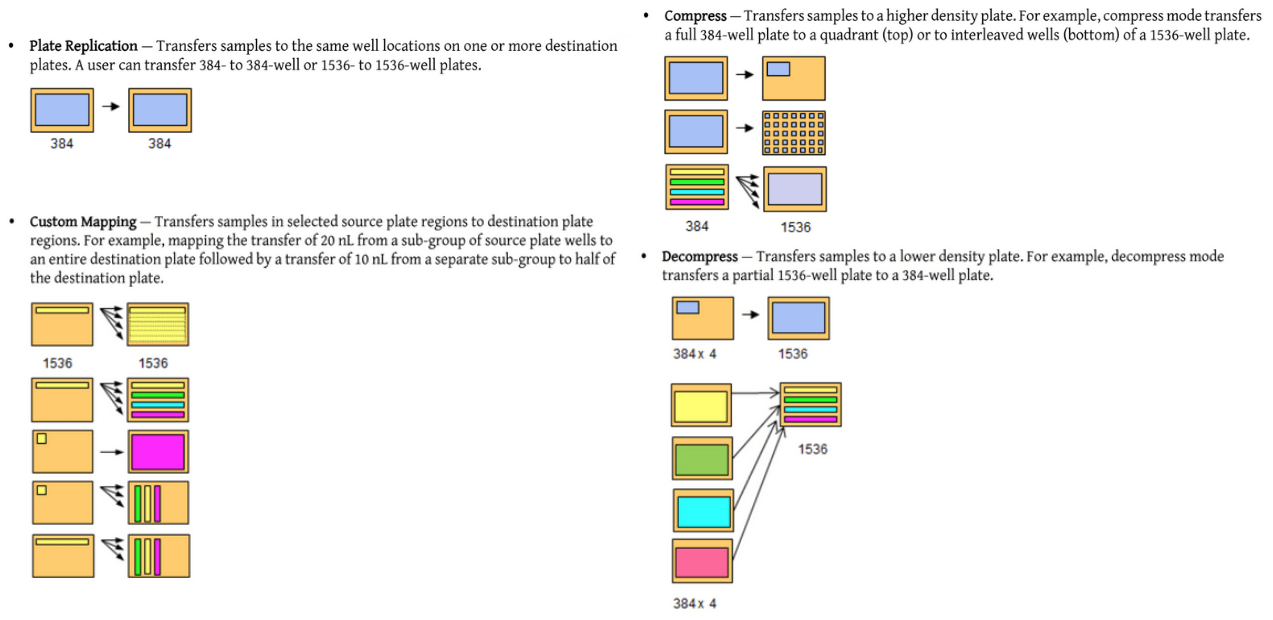
Another feature that makes acoustic dispensing attractive is the easy-to-use Plate Reformat software interface. Below are diagrams (taken from the user manual) of example operations that are especially easy to program:
When is another dispenser faster than the Echo?
- Because the Echo works on one well at a time, a multichannel pipet or pin tool is typically faster overall.
- Because it transfers one 2.5 nl droplet at a time, the bigger the volume you want, the longer it will take. Therefore a pipetting or pinning robot will be exponentially faster for larger volumes.
How can I speed up the Echo's work?
- 2.5 nl at a time sounds very slow, but keep in mind that the Echo can dispense a stream of hundreds of droplets per second. So choosing a smaller volume will speed things up, but is not the only factor to consider.
- The Echo has to refocus the sound wave for each source well, so a workflow design with fewer, larger, fuller source wells is going to be faster.
- Therefore, if you are doing several replicate assay wells of one compound, or doses of that compound, it is actually faster to have one well with all the source volume in one place, and go from there to several destination wells. The diluent for the doses is put in a separate well.
- Each time the source or destination plate has to be swapped out, the grabbers have to come in and out a door, and they have to be passed by a deionizer, which takes some time. So a workflow design with fewer in-and-out of the plates is best. This could be done by increasing the format of your plate, or putting replicates/doses of each compound onto the same plate instead of separate plates.
When does the Echo outperform other alternatives?
- The Echo is preferred for miniaturized assays i.e. 1536 well format (~5ul assay final volume).
- This is a great format for when one or more components are precious, but the detection at the end of the assay is sensitive enough to detect these small amounts (e.g. amplified by PCR).
- More wells per plate also means less plates used, less plastic waste generated.
- This can be especially good for radioactive assays or other situations where waste disposal is costly.
- The CSC's pipeting and pinning robots can be challenging to calibrate precisely enough for this format. So the Echo is prefered.
- Note that you can readily use the BioTek EL406 for bulk dispensing into 1536 format plates with the syringe modules or the 1ul peri cassette. These will have a larger dead volume than the Echo but you can have all your source liquid in one flask and save the material in the tubes at the end of the run.
- For example, you may want to pre-fill many assay plates, if your workflow typically starts with a bulk dispensing of something that can be stored long term (e.g. assay buffer, protein solution). Then thaw and use as destination plate in the Echo as needed for the smaller volume component addition in the second step.
- If you have a very concentrated stock solution and are seeking a significant dilution ratio in your assay, then the Echo's ability to transfer small droplets can void the necessity for an intermediate plate (i.e. the diluted stock plate, from which to pin or pipet), saving you time and plates, and freeing up space in your freezer that may have been taken up by such intermediate plates.
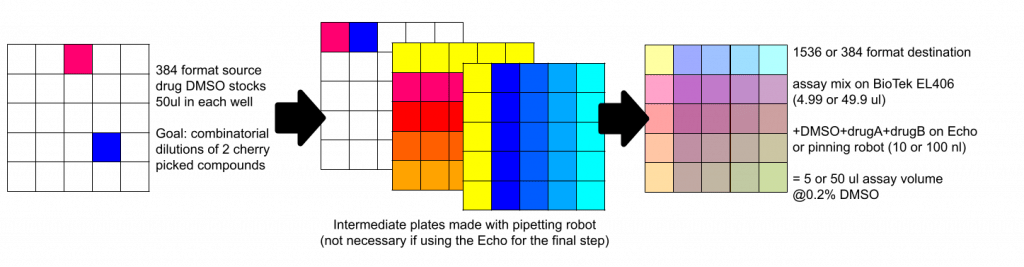
- If you want to perform complex plate map changes between the source and the destination, the Echo can be easily programmed to perform any such adjustment, again without the need for intermediate plates, saving you time and plates. Therefore it works great for combinatorial dispensing, cherry picking, pooling, creating dilution series, randomizing your plate map to remove bias (see image below), etc. For some cool ideas, see this paper from SLAS.
- Within the Echo's dedicated Plate Reformat software, you can either perform pre-programmed basic replica printing of your plate map, program a new plate map using an interactive pictographical display of the source and destination, or load a spreadsheet with the plate map specifications in a certain format.
- Unlike tip-based, syringe, and peristaltic transfer mechanisms, it is more accurate the smaller the volume transferred.
- If you plan to dispense larger volumes, consider that the error is magnified with each 2.5nl droplet.
- Imagine pipetting or pinning 100 times into the same well – eventually the error would magnify to an unacceptable level.
- Since ADE is so accurate, 250 nl (100 droplets transferred) is still expected to be dispensed accurately. However, 2.5 ul (1,000 droplets transferred) is pushing the limit of what could be feasibly accurate.
- Luckily tips are really good at transferring in the ul ranges. So acoustic dispensing is a good compliment to tip-based technologies for the different parts of your workflow.
- Consider the error rate when performing serial dilutions. At each dilution step, the error is compounded. For example, consider a 1:10 dilution that has a 10% error such that it is effectively a 1:9 dilution. In a 4 point serial dilution, instead of 1, 0.1, 0.01, 0.001, the concentrations would be 1, 0.11, 0.012, 0.0014. This is not true for the Echo, which performs direct dilution. This is explored further in the figure below:
image credit: Russ DuChene
Source plate
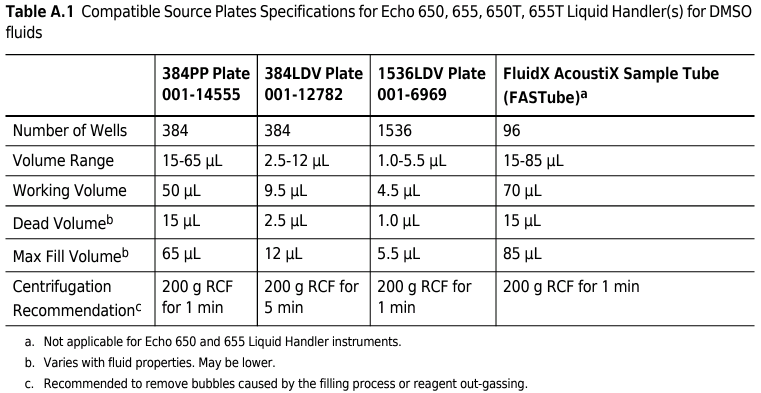
- Must be an Echo-qualified plate.
- Only Labcyte brand plates give optimal volume transfer accuracy.
- Will be either 384 or 1536 format.
- Must contain a volume between a specific range, that varies depending on the plate type and the ingredients of the liquid.
- Must be spun down very hard to make a perfect flat surface.
- Cannot have any metal on it anywhere e.g. no remnants of a foil seal.
Destination plate
- Works best if the plate is dry.
- It will be flipped upside down by the plate loading arm, and dispensed into at this upside down orientation. Therefore there is a limit to how much volume of liquid can be in the well at the start and at the end of the liquid transfer. Under no circumstances can any liquid be allowed to drip out of the wells into the Echo device! There is no cleaning procedure for that, and there are complex mechanical and electronic components below the plates. The allowable volume depends on your well dimensions, well substrate and coatings, and liquid contents (hydrophilic coatings and surfactants make it worse).
- It cannot have any metal.
- Can be almost anything that fits into a microplate sized holder and can be gripped by the razor gripper:
- Could be a single well with a thin layer of agar for dispensing microbes to form colonies. Or a lawn of microbes for dispensing drugs to see cytotoxicity.
- Could be mounted tissue slices for delivering compounds or dyes with XY resolution.
- Could be a nitrocellulose or other membrane for dispensing samples to test with a dot blot using antibodies
- Could be a tray for crystallography: see a variety of examples from Beckman Coulter here and a published example here. Also, you can use the survey function to detect crystal formation in crystal trays. See publication here.
Mass Spectrometry
- The destination plate could be a specially made MALDI plate for mass spec. Examples in the literature:
- Beeman et al. 2017 in SLAS, supplemental info see figure S1 for the special MALDI plate adapter.
- "Prior to spotting, MALDI targets (polished steel plain 1.0 inserts, Bruker Daltronics 1833280) were cleaned using the following steps: (1) 100% TFA, (2) water (Baker), and (3) allowed to dry at room temperature…The plain steel MALDI target plate on the 3D printed adapter (Suppl. Fig. S1) was introduced into the Echo 550 as a destination plate. Seventy-five nanoliters of the assay and matrix mix was transferred to the target plate in a 1536-well grid format…The rationale for using the Echo liquid handler was its common use in HTS workflows, its routine integration in our well-established HTS system (Suppl. Fig. S3), and its proven reliability." [continued in the Results/Discussion section is a whole lot more about the Echo.]
- Hruba et al. 2022 in Biomedicine & Parmacotherapy. Click here for an image of their home-made MALDI plate for use in an Echo.
- "We designed and 3D printed a holder for the 1536-well MALDI plates (Fig. 1), inspired by standard source plates for the Echo 550 but significantly lighter than usual metal MALDI holders. Owing to its compatibility with Echo, we were able to automatically transfer samples with matrix to the 1536-well MALDI plates, speeding up the process considerably. …Compared to other studies, uniqueness of our study is in the connection between non-contact echo-acoustic liquid handling instrumentation (ECHO 550) for reaction preparation and spotting of the samples on the MALDI plate and MALDI-TOF mass spectrometer which has made our assay more automated and allows us to work with very small volume of enzymes, substrates and analysed compounds (total reaction volume is 2.593 µl) with low risk of sample cross-contamination. Advantage of this method was its simplicity of implementation, accuracy in the identification of the substrate and reaction product by specific molecular weight detected by highly sensitive MALDI-TOF mass spectrometry and its suitability for high throughput screening. Samples were mixed automatically with minimal enzyme and substrate consumption and the 1536-well format allowed large numbers of samples to be analyzed quickly (3–4 h for the acquisition of 1536 data points). Unlike other methods, the evaluation of enzymatic activity was based on direct detection of the substrate and the reaction product. Thus, there was no need to use any chromogenic substrates or labeled radionuclides. The reaction mixture consists only of the enzyme, peptide substrate and reaction buffer (optionally, an inhibitor may be present) and therefore the interference with the assay is minimized."
-
Chin et al. 2015 in SLAS Technology. "The indium tin oxide (ITO)–coated glass slides and a MTP Slide Adapter II were obtained from Bruker Daltonics (Billerica, MA)…Acoustic sample deposition was performed using the Acoustic Transfer System (ATS-100) Biosero (San Diego, CA) and TransferTrack software for plate map generation from the 384-well source plate onto the glass slide. Samples were prepared on the ITO-coated glass slides by first spotting the matrix (5 nL), drying the slide under vacuum for 2 h, spotting the compounds (20 nL), and then finally drying under vacuum before analysis. "
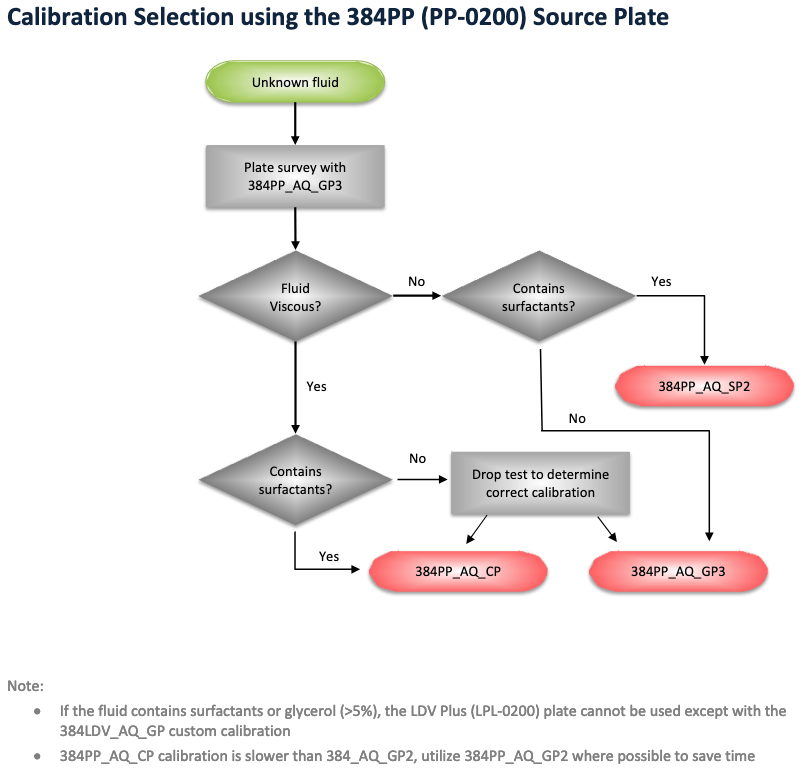
The Echo is programmed with various calibrated settings to choose from. The dispensing will only be accurate if you select the appropriate settings for your source plate type and your sample contents. Click here for a table of settings. The flowchart below that explains how to go about selecting the settings for a plate that you're not sure about.
Interface and integration: The Echo and its PC are connected through the Explorer workstation. For stand-alone, walk-up use, the Echo is operated by Plate Reformat software (not to be confused with Plate Works software that controls the Explorer!). Since it is integrated with a robot arm, there is no stacker on the Echo itself.
(stay tuned for more details being added here! about plate types and ordering information, volumes for different liquids, etc)
Comparison with other Echo models: Compared with the older 500 series models, ours can dispense 10x smaller volumes (25 versus 2.5 nl droplets) and can dispense DMSO (the 500 series can only dispense aqueous liquids). Compared with the T model, ours does not accept sample tubes, only microplates.
We do not currently offer:
- the following that are optional packages could be acquired with your contribution:
- additional software packages from Beckman Coulter to make it easier to program complex dispensing tasks. There are packages specifically for cherry picking and making dilution series.
Resources:
- Echo Plate Reformat software brief user instructions from Beckman Coulter – a good starting point! (4 pages PDF)
- Echo calibration selection describes what settings to choose for DMSO, glycerol, protein, aqueous, etc. – the compatibilities vary depending on plate type! (21 pages PDF)
- Echo full Plate Reformat user manual from Beckman Coulter (132 pages PDF)
- Echo Plate Audit software brief user instructions from Beckman Coulter – for surveying the volume and % DMSO of your source plate (5 pages PDF)
- Echo full Plate Audit user manual from Beckman Coulter (110 paged PDF)
- Echo user instructions from Beckman Coulter (174 pages PDF)
- Echo user instructions from Perkin Elmer – for when using it via PlateWorks on the Explorer workstation. Includes “pick list” information. (12 pages PDF)